Cell Therapy
Research & Innovation
Cell Therapy
Cell Therapy
On June 18, 2019, we established the cell therapy center in China Medical University Hospital to promote clinical services related to the Regulations of special Medical Techniques, and actively develop and launch a number of multi-center clinical trials related to cell therapy. The clinical service operation of this cell therapy center has always been the learning object of many domestic medical institutions, and the R&D team is constantly innovating and actively conducting multi-center clinical trials of Phase I or Phase II, in order to solve the unmet clinical needs. In addition, we have also set up the international collaboration with Prof. Yu-Hua Tseng in Harvard University, Boston for BAT men, Prof. Hiroshi Kawamoto in Kyoto for iPS-TCRT, and Prof. Raymond Ching-Bong Wang in Melbourne for PD-1/TK iPS to develop advanced cell therapy for both cancer and regenerative medicine.
Main Product
Dendritic cell, DC therapy cell therapy
Product Introduction
The principle of dendritic cell (DC) therapy lies in harnessing the unique ability of DCs to present cancer antigens to T lymphocytes, enabling the immune system to recognize and eliminate tumor cells. Tumor antigens are first extracted—typically from surgical specimens—and cultured. DCs are then isolated and processed in vitro to create DC vaccines that carry these tumor antigens. Once injected into cancer patients, these vaccines stimulate and activate cytotoxic T lymphocyte responses, thereby inhibiting tumor growth.
Dendritic cell immunotherapy has thus been widely recognized as a promising adjunctive treatment for cancer. In our approach, after obtaining and culturing tumor tissue, we further apply irradiation treatment. Previous studies have shown that this strategy not only suppresses tumor cell proliferation and increases antigen purity, but also enhances the cytotoxic capacity of CD355⁺CD4⁺ T cells against cancer cells.
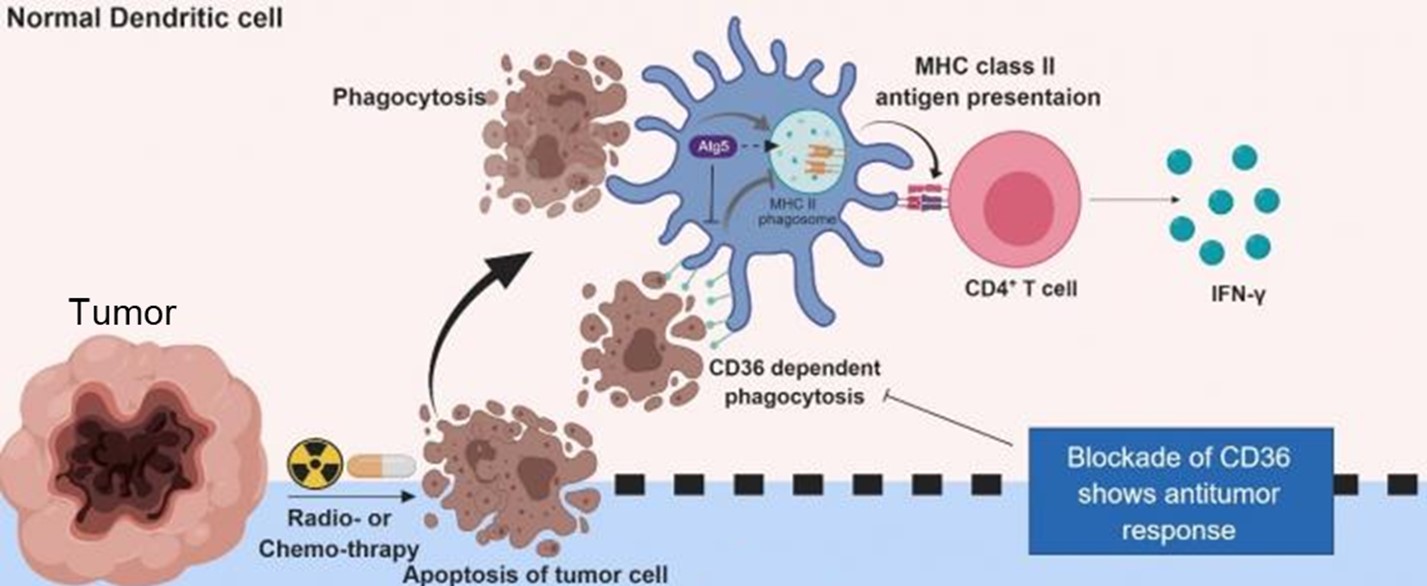
DC cells can increase the cytotoxic ability of T cells.
.jpg)
Indications:
-
Solid cancer stages I to III that are unresponsive to standard treatments:
First to second grade low-grade gliomas and third grade high-grade gliomas, liver cancer, epithelial ovarian cancer, pancreatic cancer, prostate cancer, head and neck cancer, breast cancer, colorectal cancer. -
Stage 4 solid cancer:
Glioblastoma multiforme and subsequent brain tumors, liver cancer, epithelial ovarian cancer, pancreatic cancer, prostate cancer, head and neck cancer, breast cancer, lung cancer, and colorectal cancer.
Clinical outcomes
.jpg)
Main Product
Dendritic cell-Cytokine induced killer cell-WT1,DC-CIK(WT1) cell therapy)
Product Intro.
Dendritic cell combined cytokine-induced killer cell therapy targeting WT1 (DC-CIK(WT1)) involves the in vitro co-culture of dendritic cells (DCs) and cytokine-induced killer (CIK) cells as a combinational treatment for cancer patients.
In terms of its therapeutic mechanism, dendritic cells are professional antigen-presenting cells. Mature DCs can present WT1 peptide tumor antigens via the MHC-I pathway, effectively counteracting the immune evasion mechanisms employed by tumor cells.
While CIK cells exhibit broad-spectrum cytotoxicity against tumor cells, their co-culture with DCs facilitates the presentation of WT1 peptide antigens to CIK cells. This interaction leads to enhanced activation of CIK cells and strengthens their tumor-specific cytotoxic effects.
.jpg)
The only DC-CIK(WT1) cell therapy product approved under the “Regulations Governing the Application or Use of Specific Medical Techniques or Examinations, or Medical Devices”.
.jpg)
Indications:
Stage 4 solid cancer:
Breast cancer, colorectal cancer.
Clinical outcomes
.jpg)
Main Product
Cytokine induced killer cell ,CIK cell therapy
Product Intro.
CIK cells are a special group of immune cells composed of various populations, including T cells, NK-T cells, and NK cells. They are highly safe, exhibit no restrictions related to MHC (major histocompatibility complex) molecules, and possess strong anti-tumor activity. CIK cells can boost the immune system and enhance overall immunity, particularly in patients who have undergone surgery, radiotherapy, or chemotherapy. They are effective in eliminating residual micrometastatic lesions and in preventing cancer cell recurrence and metastasis. Clinical studies have confirmed that CIK cells demonstrate significant efficacy when used in combination with cancer drugs.
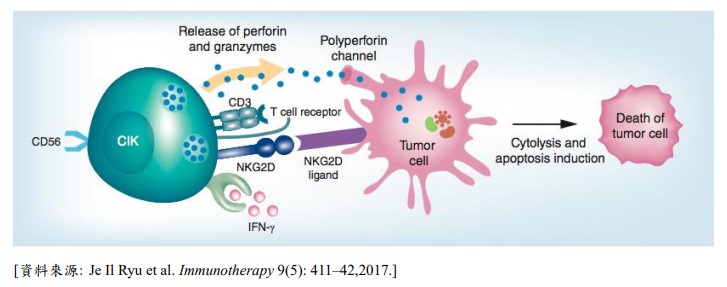
CIK has good results against cancer.
.jpg)
Indications:
-
Solid cancer stages I to III that are unresponsive to standard treatments:
Epithelial ovarian cancer, lung cancer, liver cancer, breast cancer, colorectal cancer, pancreatic cancer. -
Stage 4 solid cancer:
Glioblastoma multiforme, epithelial ovarian cancer, lung cancer, liver cancer, breast cancer, colorectal cancer, pancreatic cancer, urothelial cell cancer. -
Hematological malignancies: Hodgkin's lymphoma, B-cell non-Hodgkin's lymphoma, acute myeloid leukemia, acute lymphoid leukemia, chronic myelogenous leukemia, chronic lymphocytic leukemia, multiple myeloma.
Clinical outcomes
.jpg)
Main Product
Gamma-Delta T,GDT cell therapy
Product Intro.
GDT cells are T lymphocytes with similar innate immunity. They are characterized by T cell receptors composed of gamma and delta chains. They are widely distributed in peripheral blood and mucosal tissues. They can quickly identify pathogens and further initiate adaptive immunity. They are The first line of immune defense. Activated GDT cells have diverse abilities, including the ability to eliminate infected cells or tumor cells, produce cytokines and chemokines, antigen presentation and regulatory capabilities. Therefore, GDT cells are useful for controlling human infections, allergies, autoimmunity and cancer. The occurrence and process both play an important role. The results of multiple clinical studies have also confirmed that GDT cells are effective when used in combination with cancer drugs.
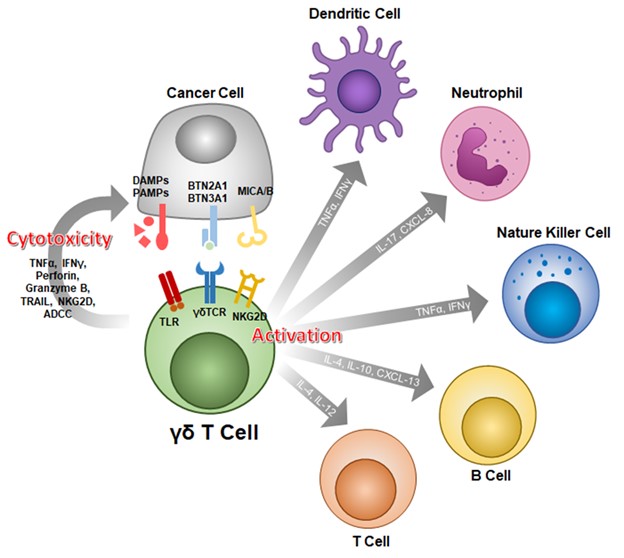
GDT attacks cancer cells by recognizing multiple antigens.
.jpg)
Solid Cancer Stage IV;
Breast, Lung, Colorectal Cancer, Renal cell carcinoma, Prostate cancer, Pancreatic cancer, Gastric cancer, Cholagiocarcinoma.
Certification/Award


Certification/Award
|
2019, th16 National Innovation Award |
2020, th17 National Innovation Award |
|
2021, th18 National Innovation Award |
2020, Future Tech Award |
|
2022, th19 National Innovation Award |
Contact Information
Unit/Telephone (including extension)/E-Mail
Cell therapy canter / 04-22052121 #15190、15196、15197 / cmuh.10MB@tool.caaumed.org.tw





