Department Introduction
Translational Medicine Research Center | Excellence
:::
Excellence
The center is mainly concerned with the molecular mechanism of the neurotransmitter receptor transporting in neurons, giving dynamic expression and regulation on the membrane surface, and its role in the occurrence and development of nervous system diseases. It identifies key molecules that cause pathological changes in the disease, and then designs specific drugs for central nervous system diseases such as stroke, epilepsy, drug addiction, and others.

The human brain nerve relies on the information element to transmit information, and if an obstacle occurs, it will get ill. In the past, if there is a disorder in one region of the brain, other undiseased areas must be treated with drugs as well. The new method we have invented is that the drug can find the lesion area by itself, and the normal cells are not affected in the process of treatment.
Molecularly Targeted Therapy
The death of nerve cells after apoplexy can be attributed to a variety of factors. Excessive activation of the NMDA receptor is one of the main causes of neurotoxicity. As NMDA receptors are involved in many normal brain functions, drugs that act on NMDA receptors for long periods of time are seldom applied in clinical medicine due to their side effects and time limits for medication use. After nearly ten years of research on the NMDA receptor, we have made a breakthrough in the field.
The N-methyl-D-aspartate (NMDA) receptor is an ion channel. When two nerve cells are stimulated at the same time, the response intensity of the synapses between the two cells will be strengthened. The effect of long-term potentiation (LTP) can last for several hours to a few days, and the excited neurons will produce a nerve conduction molecule called glutamate.
The LTP of the synapse is found in several specific parts of the brain, especially in the hippocampus of the medial temporal lobe, which is the basic mechanism for learning and memory. The NMDA receptor receives the glutamic acid, polarizing the cell membrane, producing the calcium ion flow, and then starts the LTP. In short, the NMDA receptor controls the initiation of LTP in the hippocampus.
Our study found that the NMDA receptor with different subunits after stroke has specific electrophysiological and pharmacological properties, and it combines with different connexins, and even its distribution varies according to space and time. Only drugs that work on specific NMDA subunits can reduce the area of post-stroke nerve injury.
After screening the activating transcription factors after stroke, we further determined the important role of SREBP1 and its repressor protein INSIG1 in the NMDA subunit downstream signaling system. The polypeptide-based drugs we designed accordingly have a strong neuroprotective effect on stroke animals.
These research results have been published in international authoritative journals Science, Nature Medicine, Journal of Neuroscience, PNAS, and Nature Neuroscience. We are also dedicated to the further transformation of new drugs for cerebral apoplexy into clinical therapy.
The accompanying drawings are as follows:

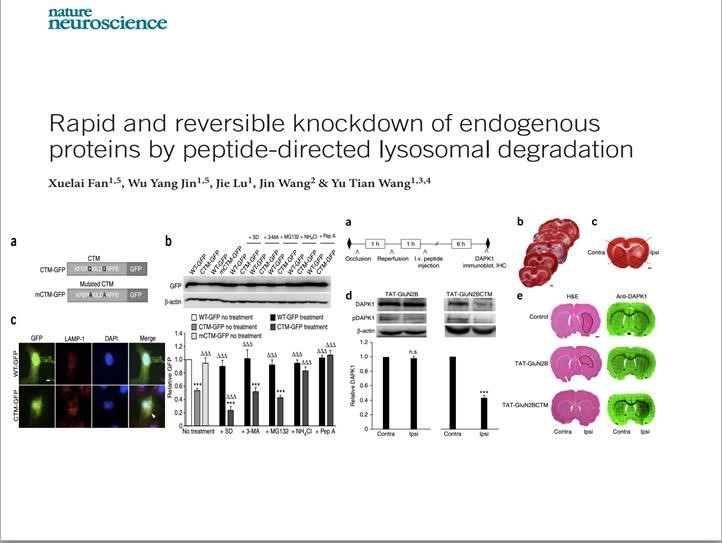
Figure 1 Director Yu-tian Wang published in 2015 a paper titled “Rapid and reversible knockdown of endogenous proteins by peptide-directed lysosomal degradation”

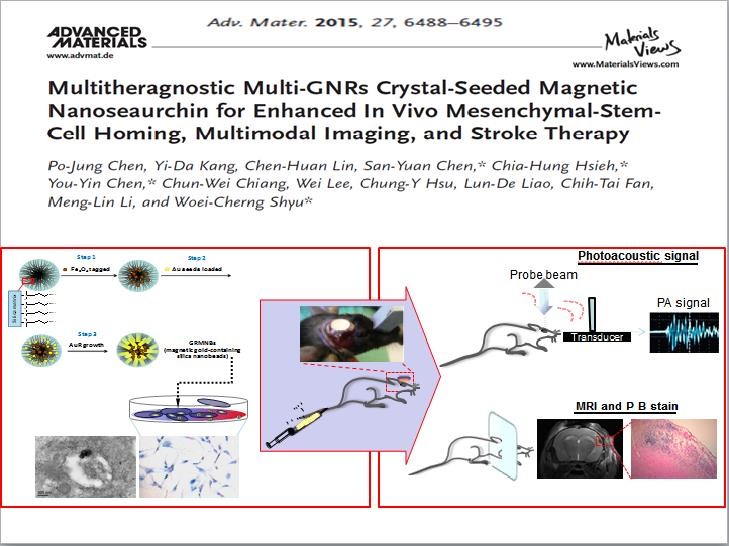
Figure 2 Deputy Director Woei-cherng Shyu published in 2015 a paper titled “Multitheragnostic Multi-GNRs Crystal-Seeded Magnetic Nanoseaurchin for Enhanced In Vivo Mesenchymal-Stem-Cell Homing, Multimodal Imaging, and Stroke Therapy”
▲
